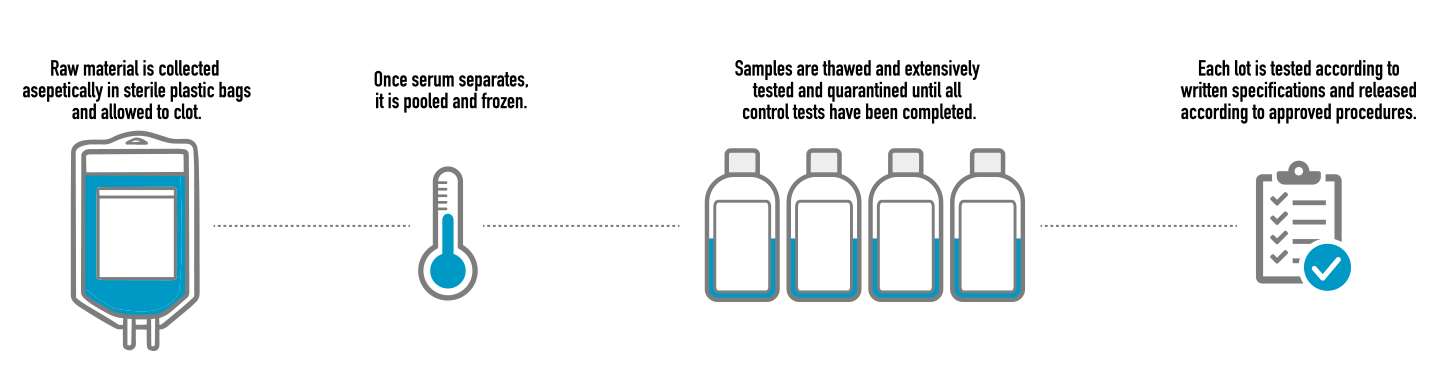
Schematic diagram of a protocol for effective inactivation of NiV in... | Download Scientific Diagram

A tissue culture infectious dose-derived protocol for testing of SARS-CoV-2 neutralization of serum antibodies on adherent cells: STAR Protocols

Effective chemical virus inactivation of patient serum compatible with accurate serodiagnosis of infections - Clinical Microbiology and Infection

Quantitative In-Vitro Diagnostic NMR Spectroscopy for Lipoprotein and Metabolite Measurements in Plasma and Serum: Recommendations for Analytical Artifact Minimization with Special Reference to COVID-19/SARS-CoV-2 Samples | Journal of Proteome Research

EVs analysis in the COVID-19 era: insights on serum inactivation protocols towards downstream isolation and analysis | bioRxiv
Protein denaturation caused by heat inactivation detrimentally affects biomolecular corona formation and cellular uptake - Nanoscale (RSC Publishing)
Extraction-free protocol combining proteinase K and heat inactivation for detection of SARS-CoV-2 by RT-qPCR | PLOS ONE

EVs analysis in the COVID-19 era: insights on serum inactivation protocols towards downstream isolation and analysis | bioRxiv

Viruses | Free Full-Text | Heat Inactivation of Different Types of SARS-CoV-2 Samples: What Protocols for Biosafety, Molecular Detection and Serological Diagnostics? | HTML
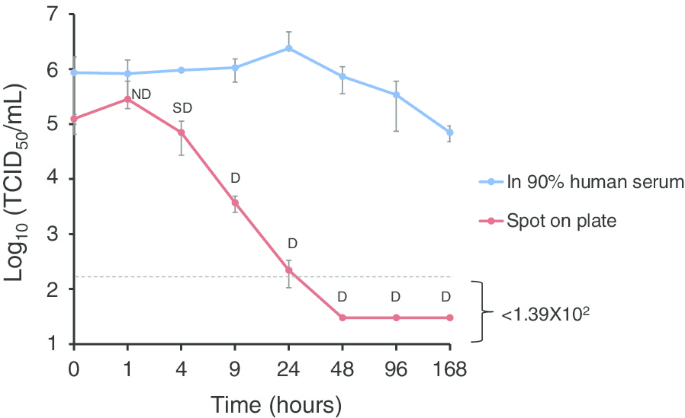
Effective inactivation of Nipah virus in serum samples for safe processing in low-containment laboratories | Virology Journal | Full Text

A tissue culture infectious dose-derived protocol for testing of SARS-CoV-2 neutralization of serum antibodies on adherent cells: STAR Protocols

Influenza Virus Inactivation for Studies of Antigenicity and Phenotypic Neuraminidase Inhibitor Resistance Profiling | Journal of Clinical Microbiology
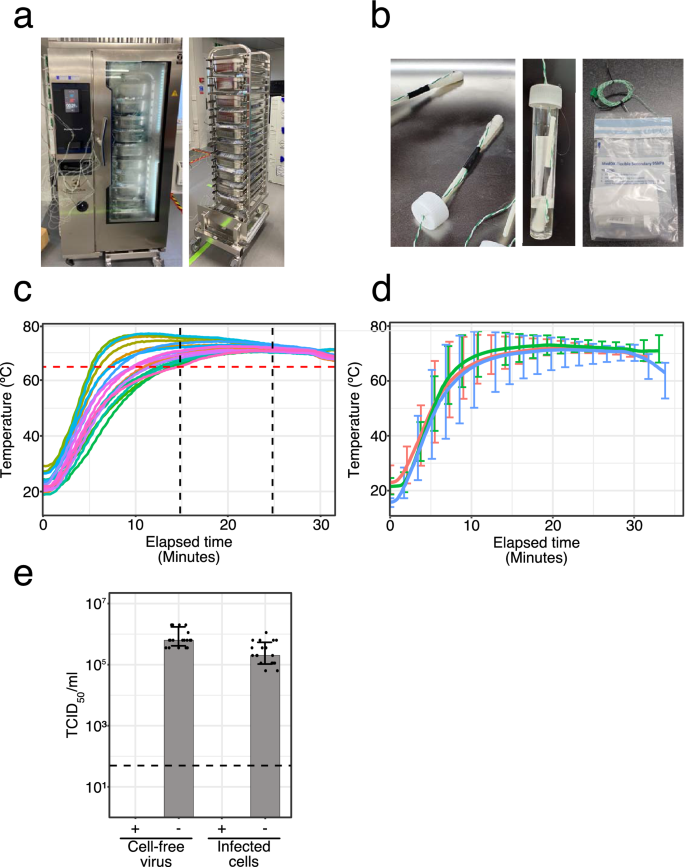
Heat inactivation of clinical COVID-19 samples on an industrial scale for low risk and efficient high-throughput qRT-PCR diagnostic testing | Scientific Reports