![Polymers | Free Full-Text | [Rh(L-alaninate)(1,5-Cyclooctadiene)] Catalyzed Helix-Sense-Selective Polymerizations of Achiral Phenylacetylenes | HTML Polymers | Free Full-Text | [Rh(L-alaninate)(1,5-Cyclooctadiene)] Catalyzed Helix-Sense-Selective Polymerizations of Achiral Phenylacetylenes | HTML](https://www.mdpi.com/polymers/polymers-10-01223/article_deploy/html/images/polymers-10-01223-g001.png)
Polymers | Free Full-Text | [Rh(L-alaninate)(1,5-Cyclooctadiene)] Catalyzed Helix-Sense-Selective Polymerizations of Achiral Phenylacetylenes | HTML
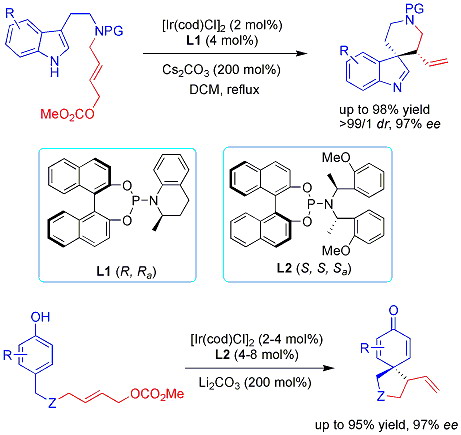
Ir-Catalyzed Asymmetric Allylic Dearomatization Reactions---State Key Laboratory of Organometallic Chemistry 345 Lingling Lu, Shanghai
![Ir(COD)Cl]2 as a Catalyst Precursor for the Intramolecular Hydroamination of Unactivated Alkenes with Primary Amines and Secondary Alkyl- or Arylamines: A Combined Catalytic, Mechanistic, and Computational Investigation | Journal of the American Ir(COD)Cl]2 as a Catalyst Precursor for the Intramolecular Hydroamination of Unactivated Alkenes with Primary Amines and Secondary Alkyl- or Arylamines: A Combined Catalytic, Mechanistic, and Computational Investigation | Journal of the American](https://pubs.acs.org/cms/10.1021/ja908316n/asset/images/medium/ja-2009-08316n_0012.gif)
Ir(COD)Cl]2 as a Catalyst Precursor for the Intramolecular Hydroamination of Unactivated Alkenes with Primary Amines and Secondary Alkyl- or Arylamines: A Combined Catalytic, Mechanistic, and Computational Investigation | Journal of the American
![Catalysts | Free Full-Text | Strong Solvent Effects on Catalytic Transfer Hydrogenation of Ketones with [Ir(cod)(NHC)(PR3)] Catalysts in 2-Propanol-Water Mixtures | HTML Catalysts | Free Full-Text | Strong Solvent Effects on Catalytic Transfer Hydrogenation of Ketones with [Ir(cod)(NHC)(PR3)] Catalysts in 2-Propanol-Water Mixtures | HTML](https://www.mdpi.com/catalysts/catalysts-10-00017/article_deploy/html/images/catalysts-10-00017-g001.png)
Catalysts | Free Full-Text | Strong Solvent Effects on Catalytic Transfer Hydrogenation of Ketones with [Ir(cod)(NHC)(PR3)] Catalysts in 2-Propanol-Water Mixtures | HTML

Scheme 3 | Luminescent Iridium Complexes Used in Light-Emitting Electrochemical Cells (LEECs) | SpringerLink
![Reactivity of Phosphane–Imidazolium Salts Towards [Ir(COD)Cl]2: Preparation of New Hydridoiridium(III) Complexes Bearing Abnormal Carbenes - Wolf - 2008 - European Journal of Inorganic Chemistry - Wiley Online Library Reactivity of Phosphane–Imidazolium Salts Towards [Ir(COD)Cl]2: Preparation of New Hydridoiridium(III) Complexes Bearing Abnormal Carbenes - Wolf - 2008 - European Journal of Inorganic Chemistry - Wiley Online Library](https://chemistry-europe.onlinelibrary.wiley.com/cms/asset/7d35c1e7-977c-43c1-bcee-fbe7d329cb1a/mfig000.jpg)
Reactivity of Phosphane–Imidazolium Salts Towards [Ir(COD)Cl]2: Preparation of New Hydridoiridium(III) Complexes Bearing Abnormal Carbenes - Wolf - 2008 - European Journal of Inorganic Chemistry - Wiley Online Library
![Ir(COD)Cl]2 as a Catalyst Precursor for the Intramolecular Hydroamination of Unactivated Alkenes with Primary Amines and Secondary Alkyl- or Arylamines: A Combined Catalytic, Mechanistic, and Computational Investigation | Journal of the American Ir(COD)Cl]2 as a Catalyst Precursor for the Intramolecular Hydroamination of Unactivated Alkenes with Primary Amines and Secondary Alkyl- or Arylamines: A Combined Catalytic, Mechanistic, and Computational Investigation | Journal of the American](https://pubs.acs.org/cms/10.1021/ja908316n/asset/images/medium/ja-2009-08316n_0017.gif)
Ir(COD)Cl]2 as a Catalyst Precursor for the Intramolecular Hydroamination of Unactivated Alkenes with Primary Amines and Secondary Alkyl- or Arylamines: A Combined Catalytic, Mechanistic, and Computational Investigation | Journal of the American
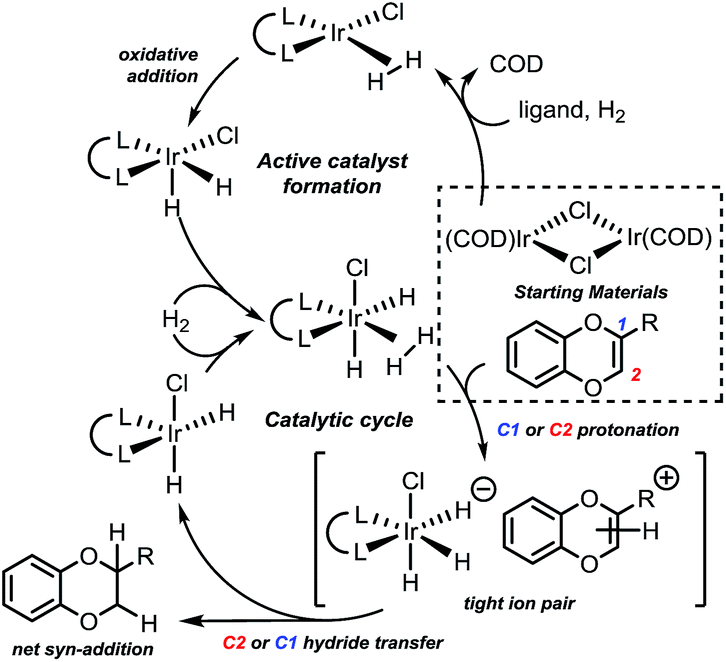
A versatile catalyst system for enantioselective synthesis of 2-substituted 1,4-benzodioxanes - Chemical Science (RSC Publishing) DOI:10.1039/C8SC05612A
![Ir(COD)Cl]2 as a Catalyst Precursor for the Intramolecular Hydroamination of Unactivated Alkenes with Primary Amines and Secondary Alkyl- or Arylamines: A Combined Catalytic, Mechanistic, and Computational Investigation | Journal of the American Ir(COD)Cl]2 as a Catalyst Precursor for the Intramolecular Hydroamination of Unactivated Alkenes with Primary Amines and Secondary Alkyl- or Arylamines: A Combined Catalytic, Mechanistic, and Computational Investigation | Journal of the American](https://pubs.acs.org/cms/10.1021/ja908316n/asset/images/medium/ja-2009-08316n_0016.gif)
Ir(COD)Cl]2 as a Catalyst Precursor for the Intramolecular Hydroamination of Unactivated Alkenes with Primary Amines and Secondary Alkyl- or Arylamines: A Combined Catalytic, Mechanistic, and Computational Investigation | Journal of the American

Figure 1 from In-Depth Study on Chloride Abstractions from (NHC)Ir(COD)Cl Complexes | Semantic Scholar
![Figure 7 from [Ir(COD)Cl]2 as a catalyst precursor for the intramolecular hydroamination of unactivated alkenes with primary amines and secondary alkyl- or arylamines: a combined catalytic, mechanistic, and computational investigation. | Semantic Figure 7 from [Ir(COD)Cl]2 as a catalyst precursor for the intramolecular hydroamination of unactivated alkenes with primary amines and secondary alkyl- or arylamines: a combined catalytic, mechanistic, and computational investigation. | Semantic](https://d3i71xaburhd42.cloudfront.net/46356b19fdd1eaa8c5903ce3fbfdabb4098eb769/9-Figure7-1.png)
Figure 7 from [Ir(COD)Cl]2 as a catalyst precursor for the intramolecular hydroamination of unactivated alkenes with primary amines and secondary alkyl- or arylamines: a combined catalytic, mechanistic, and computational investigation. | Semantic
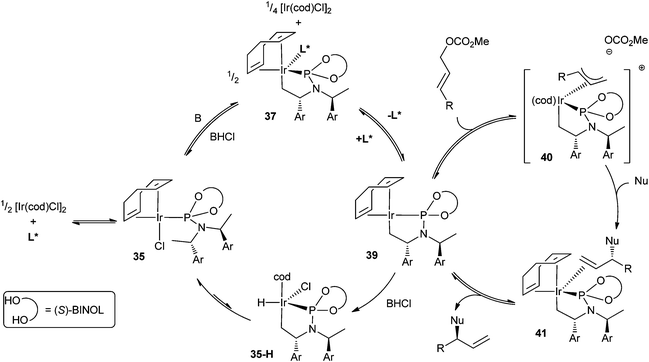
Recent advances and applications of iridium-catalysed asymmetric allylic substitution - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/C2OB07086C
![The cyclooctadiene ligand in [IrCl(COD)]2 is hydrogenated under transfer hydrogenation conditions: A study in the presence of PPh3 and a strong base in isopropanol - ScienceDirect The cyclooctadiene ligand in [IrCl(COD)]2 is hydrogenated under transfer hydrogenation conditions: A study in the presence of PPh3 and a strong base in isopropanol - ScienceDirect](https://ars.els-cdn.com/content/image/1-s2.0-S0022328X16304478-sc1.jpg)
The cyclooctadiene ligand in [IrCl(COD)]2 is hydrogenated under transfer hydrogenation conditions: A study in the presence of PPh3 and a strong base in isopropanol - ScienceDirect
![Figure 6 from [Ir(COD)Cl]2 as a catalyst precursor for the intramolecular hydroamination of unactivated alkenes with primary amines and secondary alkyl- or arylamines: a combined catalytic, mechanistic, and computational investigation. | Semantic Figure 6 from [Ir(COD)Cl]2 as a catalyst precursor for the intramolecular hydroamination of unactivated alkenes with primary amines and secondary alkyl- or arylamines: a combined catalytic, mechanistic, and computational investigation. | Semantic](https://d3i71xaburhd42.cloudfront.net/46356b19fdd1eaa8c5903ce3fbfdabb4098eb769/8-Figure6-1.png)

![Allylic amination catalyzed by [Ir(COD)Cl] 2 and L1 | Download Table Allylic amination catalyzed by [Ir(COD)Cl] 2 and L1 | Download Table](https://www.researchgate.net/profile/Alan-Goldman/publication/251330064/figure/tbl13/AS:668932327342083@1536497303136/Allylic-amination-catalyzed-by-IrCODCl-2-and-L1.png)

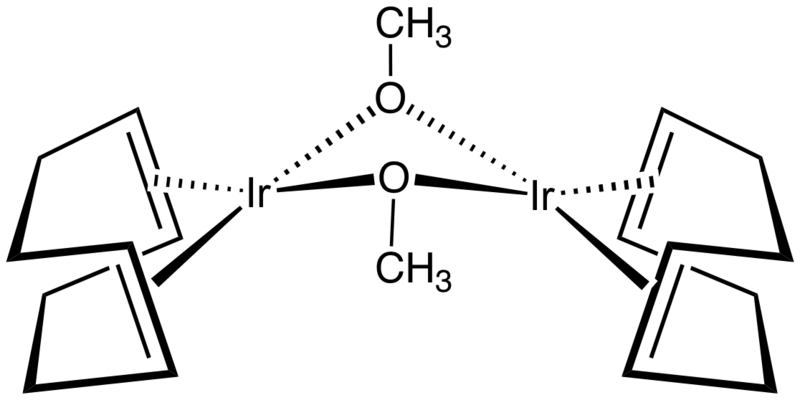
![IBIOX6]-IR-(COD)-CL - SpectraBase IBIOX6]-IR-(COD)-CL - SpectraBase](https://spectrabase.com/api/compound/ANSKGzPdbWI.png?h=300&w=416)

![IBIOXME4]-IR-(COD)-CL - SpectraBase IBIOXME4]-IR-(COD)-CL - SpectraBase](https://spectrabase.com/api/compound/JGXVXMNCehi.png?ph=true&h=300&w=416)
![Scheme 7 Allylic etherification catalyzed by [Ir(COD)Cl] 2 and a... | Download Scientific Diagram Scheme 7 Allylic etherification catalyzed by [Ir(COD)Cl] 2 and a... | Download Scientific Diagram](https://www.researchgate.net/profile/Alan-Goldman/publication/251330064/figure/fig10/AS:668932323164176@1536497302616/Scheme-7-Allylic-etherification-catalyzed-by-IrCODCl-2-and-a-phosphorodiamidite-ligand.png)