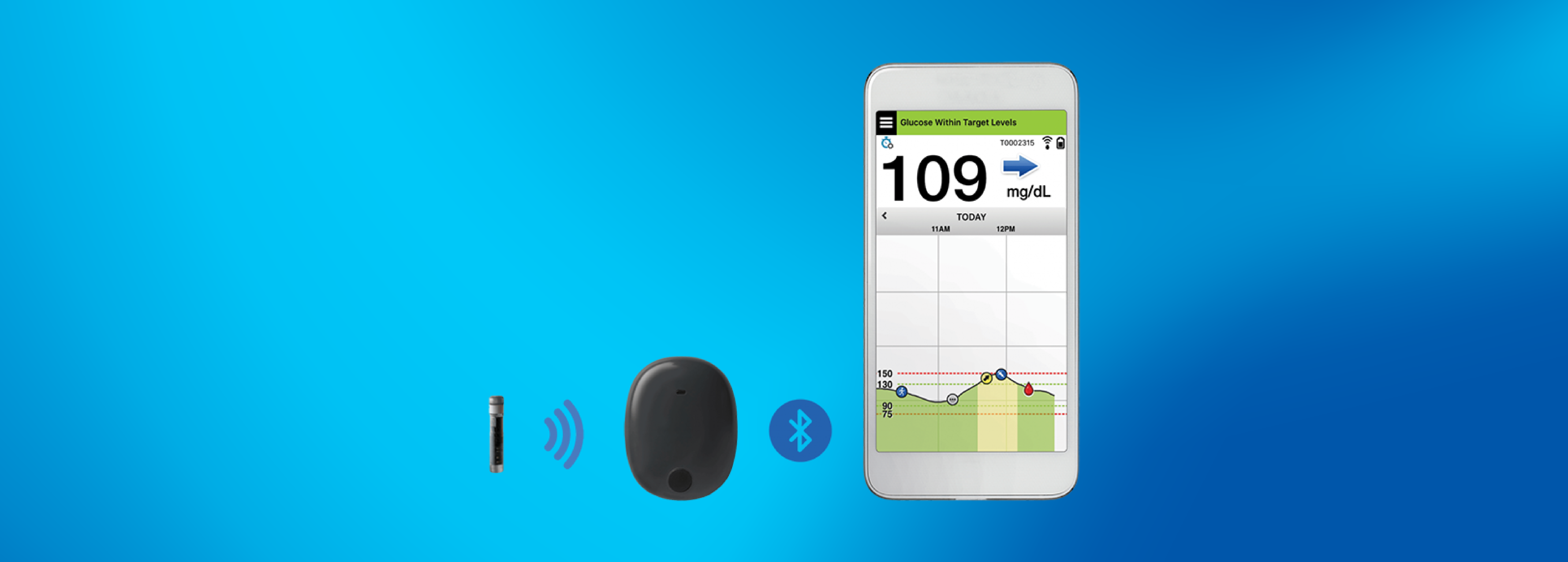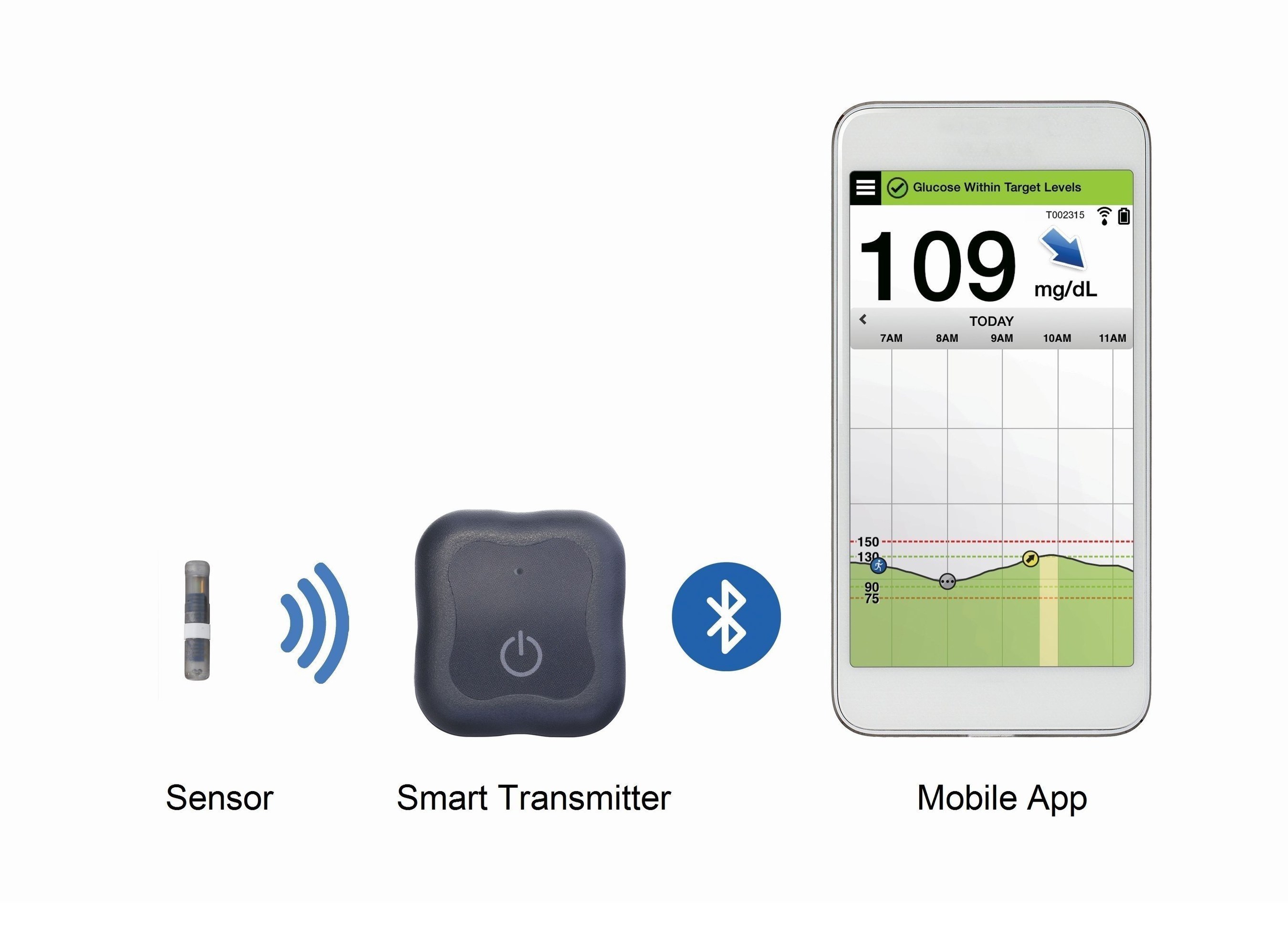
Roche Diabetes Care and Senseonics Announce Distribution Agreement for the Eversense® CGM System Introducing the First Implantable Long-term Glucose Sensor

FDA approves Eversense E3 6-month continuous glucose monitor that requires fewer fingerstick blood glucose measurements - NotebookCheck.net News

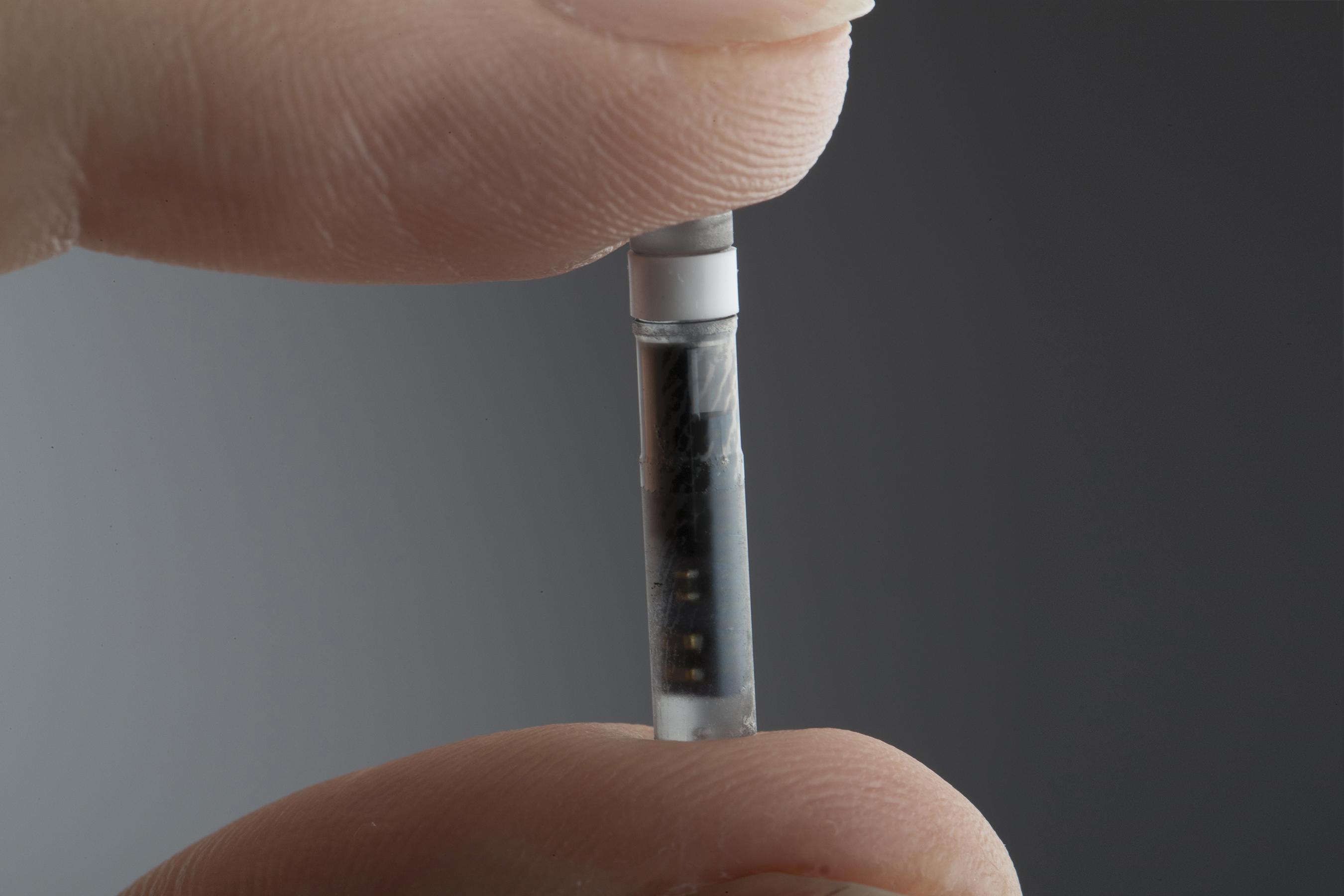
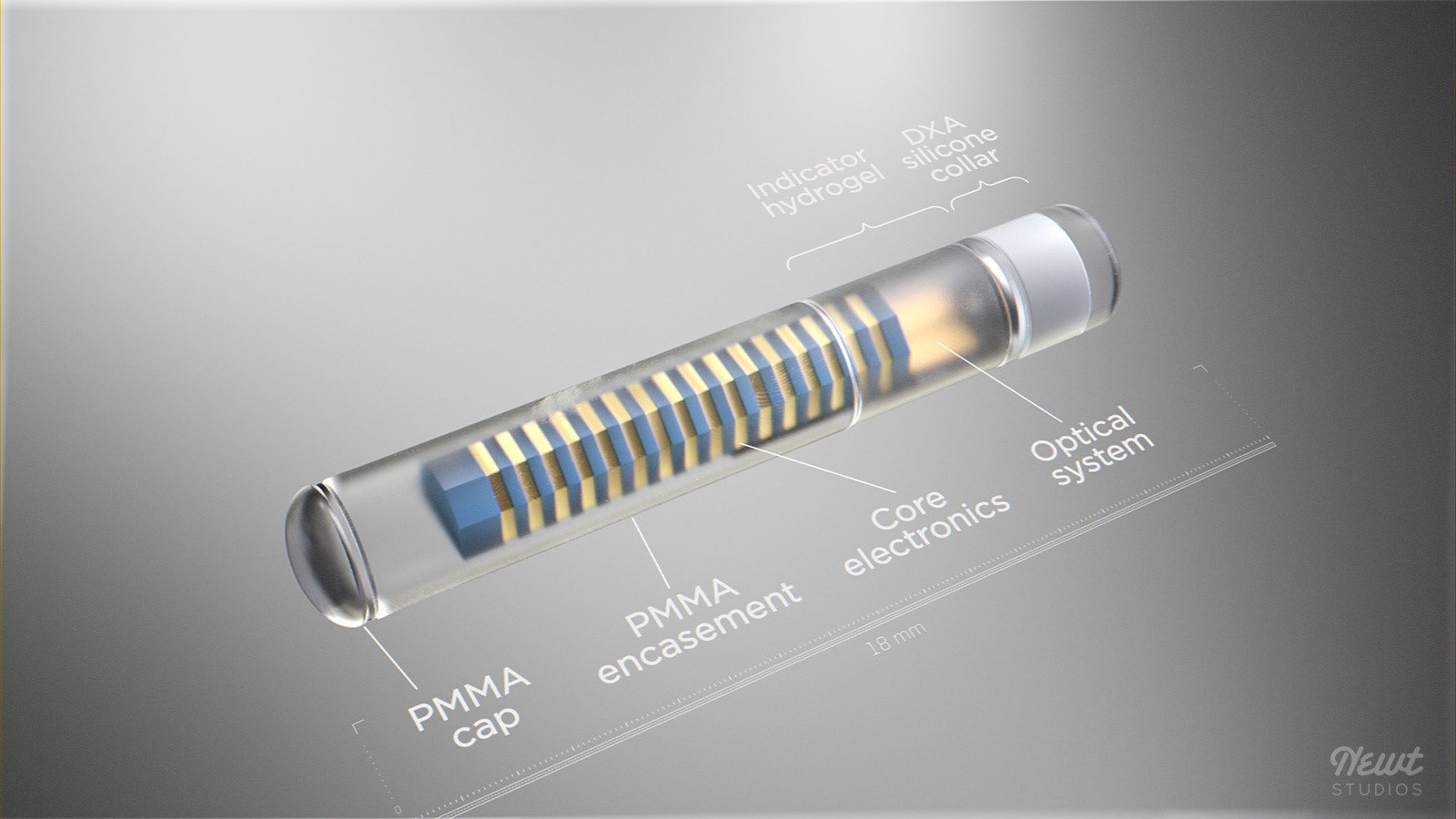
Eversense sensor. CM, centimeter; DXA, dexamethasone acetate; PMMA,... | Download Scientific Diagram

Data On Long-Term Eversense CGM System Show Sustained Accuracy In Pediatric Continuous Sensor Study | Medical Product Outsourcing